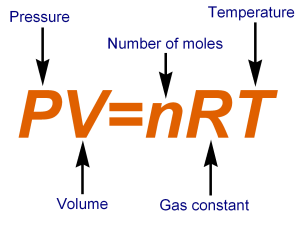
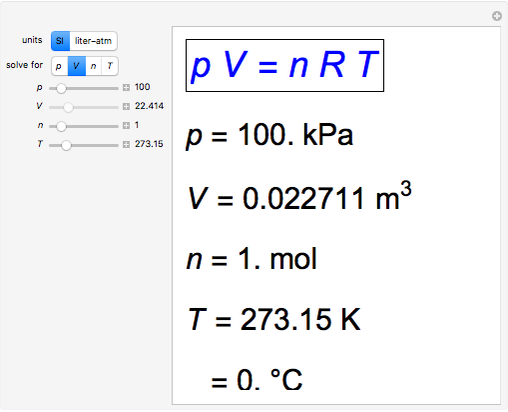
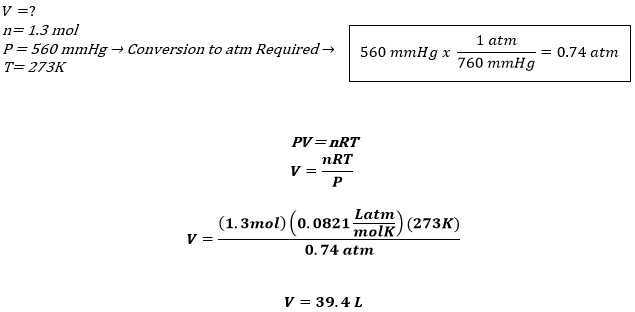
Definition: The Universal or Ideal Gas Law describes the relationship between all four properties (pressure, volume, number of moles, and temperature) as well as a gas constant called “R.” NOTE: The Ideal Gas Constant “R” has constant a value of 0.0821 L.atm/mol.K Relation: The relation between pressure (P) volume (V), number of moles (n) and…

Combined Gas Law, Definition, Formula & Example - Lesson
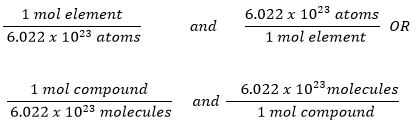
The Mole Concept: Molecules and Atoms

Equation Of State (Ideal Gas), Glenn Research Center
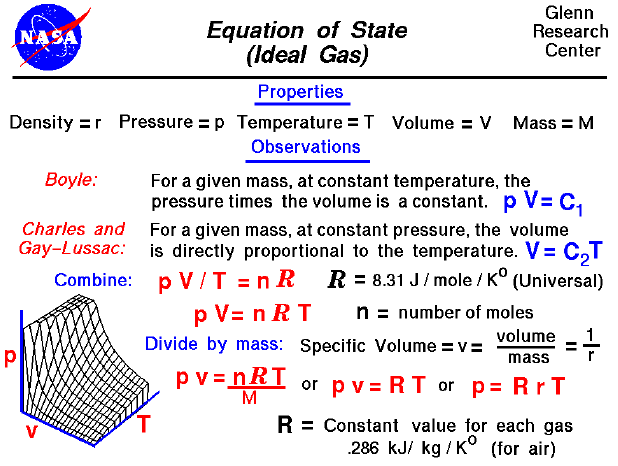
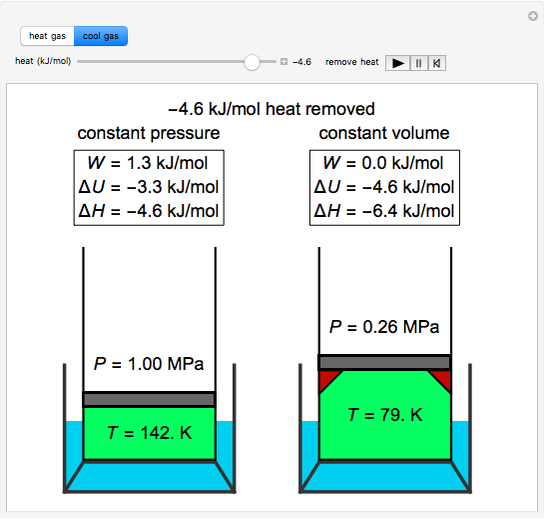
Equation of State

Ideal Gas Rulebreakers - HVAC School

ANESTHESIA EQUIPMENT AND GAS LAW REVIEW - ppt download

Ideal Gas Law: Statement, Characteristics, Formula & Problems
:max_bytes(150000):strip_icc()/200175879-001-56a12e6b5f9b58b7d0bcd67f.jpg)
An Explanation of the Ideal Gas Law

MathType on X: The gas constant “R” is defined as the Avogadro constant “NA“ multiplied by the Boltzmann constant “k”. It is mostly known for appearing in the ideal gas law and
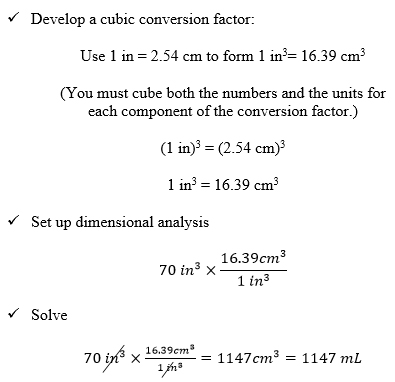
Cubic Conversions

The Mole Concept: Mass Relationships
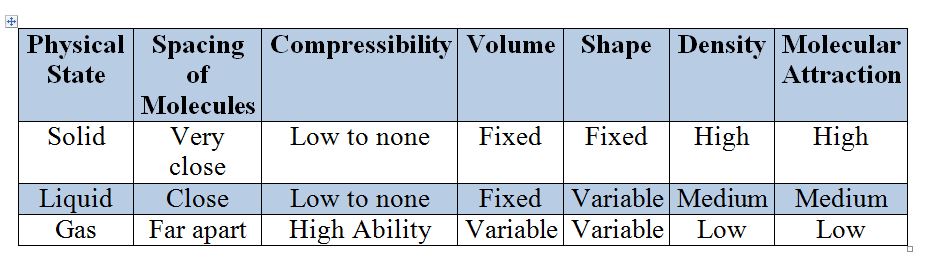
Physical States of Matter

Electromagnetic Spectrum